
Green Fluorescent Protein Purification Lab Report
Sunday, February 24, 2008
Purpose: To purify GFP from E. coli cells, this is transformed with pGLO by hydrophobic interaction chromatography.
Materials: UV Light, transformation plates, culture tubes containing the growth media LB/amp/ara (+ and -), sterile loops, shaking incubator, transfer pipette, centrifuge, microtubes, equilibration buffer, binding buffer, wash buffer, elution buffer, lysed cells containing GFP, lysozyme, freezer, chromatography column, column with HIC stationary phase, supernatant, refrigerator, foam rack, saran wrap.
Protocol Used: GFP Purification Quick Guide (Growing Cell Cultures) and Purification Phase 1-3 (Bacterial Concentration, Bacterial Lysis, and Protein Chromatography).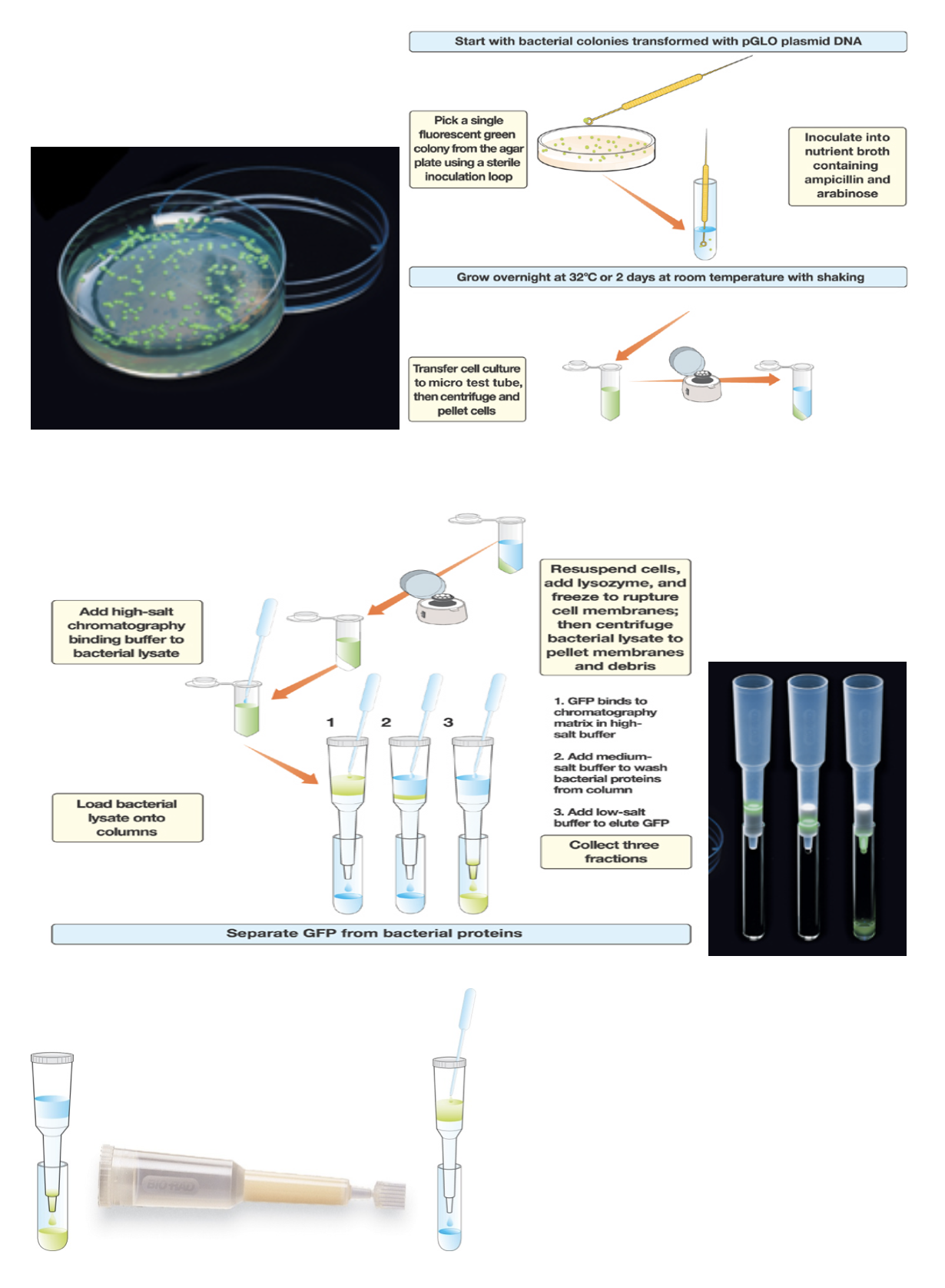
Procedure:
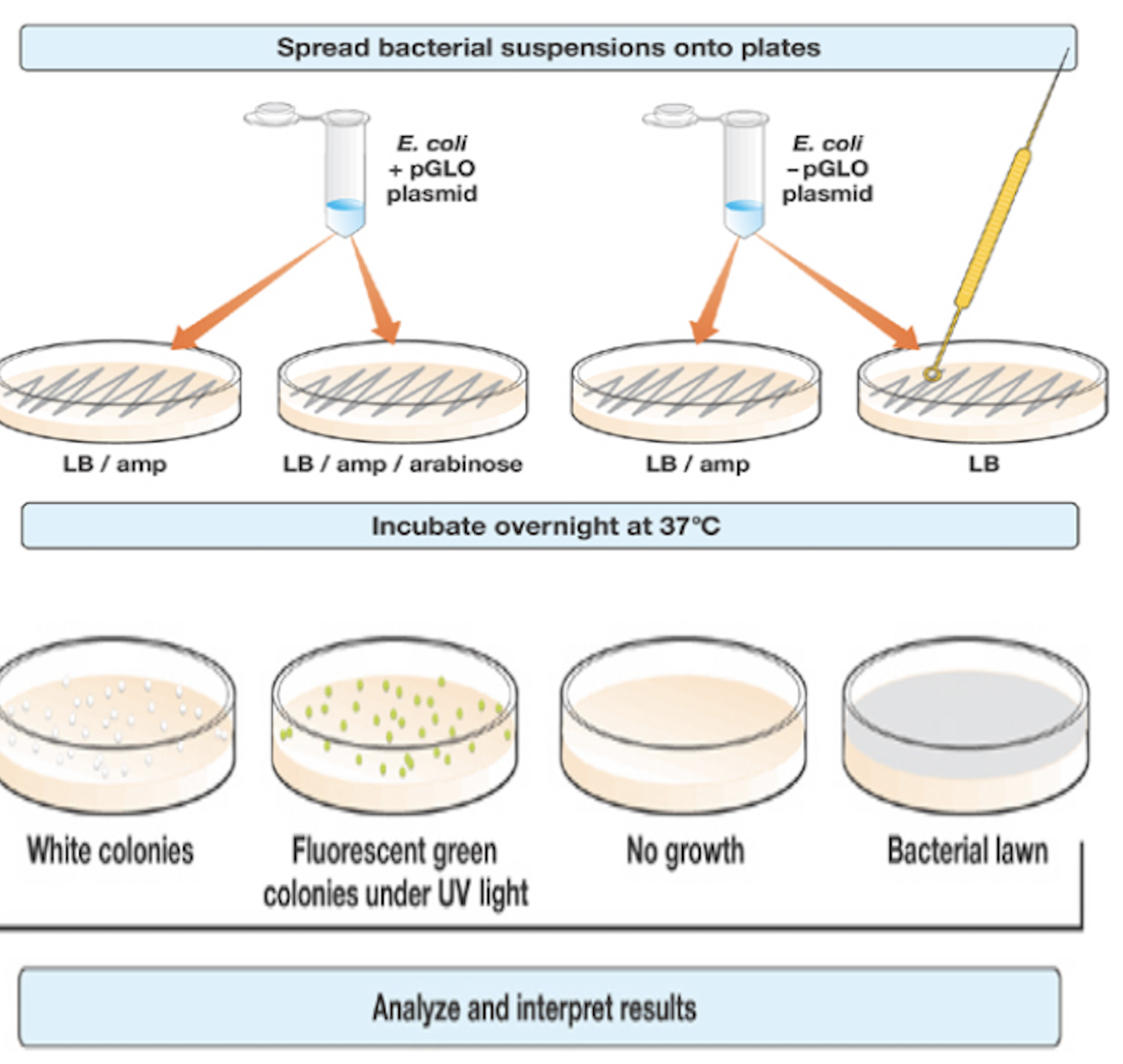
- Used a UV light to examine incubated transformation plates
- Labeled two culture tubes containing LB/amp/ara + and –
- Immersed green and white colony using a sterile loop into the + and – tubes, respectively
- Cultured tubes in shaking incubator overnight at 32 degrees Celsius
- Next day, observed cultures under UV light
- Transferred 2 ml of + liquid into a + microtube
- Spun microtube in centrifuge for 5 minutes at full speed
- Observed pellet under UV light after pouring out the supernatant
- Added 250 ml of TE solution
- Added 1 drop of lysozyme to the resuspended bacterial pellet to initiate enzymatic digestion of the bacterial cell wall
- Mixed by flicking tube
- Observed under UV light
- Stored microtube in freezer so the bacteria will rupture
- Next day, thawed microtube and placed in centrifuge, pelleting the insoluble bacterial debris
- Prepared the chromatography column, snapping off the bottom, removing the cap and allowing al the liquid buffer to drain
- Added 2 ml of equilibrium buffer
- Spun tube in centrifuge for 10 minutes
- Examined with UV light
- Transferred 250 ml of + supernatant using pipette into new microtube labeled +
- Transferred 250 ml of the binding buffer to the + supernatant
- Stored in refrigerator
- Next day, labeled 3 collection tubes 1-3 and placed in foam rack
- Placed column in collection tube 1
- Loaded 250 ml of + supernatant to the top of column
- Examined using UV light
- Transferred column to collection tube 2
- Added 250 ml of wash buffer and let flow into column
- Transferred to tube 3
- Added 750 ml of TE buffer and let flow into column
- Examined using UV light
- Examined all 3 collection tubes and notes any differences in color.
- Saran wrapped tubes and placed in refrigerator
Observations: We noted that GFP fluoresces green when exposed to UV light. It is fluorescent in many species. GFP can be analyzed in live bacteria. Many proteins fused to GFP keep their activities. Stabilizing GFP allows it to fluoresce when being purified. The bacteria were a green fluorescent color. The purification seemed to work because of the glow. We also noted that when the supernatant was dripping through, it did not glow. However, the illusion buffer displayed the glow filtering through and disappearing. Also noted, the centrifuge caused a pellet to develop at the bottom of the tube, which was to be ignored.
Results: The isolated protein caused the bacteria to grow. The protein is now a bimolecular that is usually only in jellyfish. This lab proves that we can make an organism do something if we give it the correct genetic information. After overnight incubation or refrigeration, a glow could be seen. We successfully purified GFP, causing bacteria to glow. These results are expected.
Final Comments: The salt caused the 3D structure of proteins to change so the hydrophobic regions of the protein move to the outside. Once our sample was loaded onto the matrix, the proteins stuck to the beads. GFP is a protein that can be found in the supernatant of any lysed cells.